Everyday tasks expose our hands to a great deal of risk. To protect our hands from mechanical harm, wearing safety gloves is standard practice within occupational health and safety. Yet this in itself can pose a further risk – if the material used for the safety gloves is not dermatologically tested then it can give rise to skin diseases and allergies. In this blog post, we discuss in detail the sensitising substances in safety gloves and outline how you can protect your hands against skin diseases and allergies.
Avoiding allergenic substances – guidelines
The European PPE directive and standard EN 420 expressly state that the material of gloves, the decomposition products and the substances contained must not have a detrimental effect on the health or hygiene of the user. Within professional dermatological practice, focus is also placed on the substances used in gloves as a potential cause of allergies and skin diseases. It is therefore vital to ensure that safety gloves pose no toxicological or dermatological harm. This will also reduce absences due to skin diseases and allergies, which simultaneously lowers a company’s health expenditure.

Naturally, uvex has also developed uvex safety gloves to be toxicologically and dermatologically safe, resulting in the uvex phynomic collection. The safety gloves in this series are seamless knit and have a cut protection level of 1 in accordance with EN 388 (mechanical risks). The uvex phynomic safety gloves are especially used for precision work, general assembly work and all-round tasks such as maintenance.
Dermatologically approved – the seal for healthy substances
To check that safety gloves are toxicologically safe, they are first inspected according to the international OEKO-TEX® Standard. However, the OEKO-TEX® test catalogue does not contain all the conventional substances that are used in the manufacturing process and the application of safety gloves. In particular, it does not currently cover organic solvents (dimethylformamide, secondary amines etc.) and the group of vulcanisation accelerators and the associated secondary products (carbon disulphide, nitrosamines etc.).
This is the precise focus in the uvex development processes; we offer product solutions that use no organic solvents or vulcanisation accelerators whatsoever in the manufacturing process. The result? Innovative hand protection products with hypo-allergenic properties and that pose no risk to health.

uvex is working with the proDERM® Institute for Applied Dermatological Research on a comprehensive health strategy within hand protection.
As well as toxicological safety, dermatological compatibility is another key aspect that we would like to demonstrate from an independent perspective. This is where the renowned proDERM® Institute for Applied Dermatological Research comes in, as it awards the “Dermatologically approved” quality seal.
The institute was founded in 1994 by dermatologist and allergist Prof. Klaus-P. Wilhelm and is the pioneer in independent dermatological contract research.
Step 1: Dermatological/toxicological assessment
The first step is to conduct the dermatological and toxicological assessment, which examines whether the product recipe is optimally formulated with regard to skin compatibility. Compliance with dermatological and toxicological requirements must be verified by an independent toxicologist.

Step 2: Plaster test – assessment with a focus on compatibility
The second step involves a repeated plaster test on a minimum of 30 test subjects. An initial assessment of skin compatibility is made on the basis of the maximum exposure. Parts of the glove’s knitted cuff and the coating side are applied to test subjects (of whom approx. 25% have sensitive skin or are type IV allergic).
The samples are applied occlusively for 24 hours, with a check made under medical supervision after 15 minutes, 25 hours and 48 hours to look for signs of an immediate and/or delayed reaction. Water and soap are used as reference samples for the test. The entire test is repeated three times on the same test subjects.
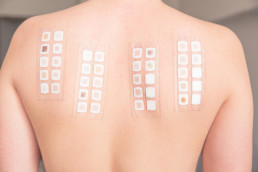
Step 3: Application-oriented study
This stage involves a dermatological application test with a dermatological and allergological evaluation. At this point, the product is used in operation over an extended period. The safety gloves were given to 30 test subjects; every day for two weeks they had to wear them the entire time that they were working. An examination for allergic reactions took place continually under medical supervision.
For the safety glove to be awarded the “Dermatologically approved” seal, the test result needs to attest to good or very good skin compatibility. Products with only average skin compatibility cannot bear the seal.
The uvex phynomic safety glove series was deemed to have very good skin compatibility and therefore gained proDERM® certification.
Faith in the fabric – DEMAG Cranes as a practical example
Of course, dermatological safety is just the first step. Ultimately, the product needs to be accepted by users – especially if they have sensitive skin.
That’s why we went behind the scenes at DEMAG Cranes – the specialist for cranes of all types. DEMAG Cranes employs many workers in its chain hoists division at Wetter in Germany – where wearing safety gloves is mandatory, of course. Safety engineer Mr Flögel had been looking for a special solution for his staff in the gear assembly department for a long time.

Fitter Detlev V. had had permanent problems with his hands for almost 7 years; he reported having constantly dry, chapped hands when wearing safety gloves and was already receiving medical attention. He had exhausted all options and tried out many products, but was unable to find a solution. In August 2011, he took part in a test for wearing our uvex phynomic foam safety glove.
“Since I’ve been wearing the gloves, my skin problems have disappeared.”
And he wasn’t alone. The entire department praised the amazing wearer comfort, the fantastic fit enabling optimum assembly work and the outstanding skin compatibility of the safety gloves.
This example shows that it is possible to use “pure” products to remedy acute problems. Many workers had had to live with minor skin problems and were thrilled that the introduction of pure products had restored normality.
Do you also suffer from a skin disease or allergy? Then you too can rely on the “Dermatologically approved” seal in future and say goodbye to rashes on your hands. We are happy to provide detailed advice about the skin compatibility of uvex safety gloves. Please contact us or leave us a comment!
This is very exciting. I have been diagnosed with touch dermatitis on my hands about 7 years ago and can not find a work glove that works for me. I’m allergic to latex, rubber, chemicals, metal, etc. just to name a few. I’m a lite vehicle mechanic on a mine site.
Any advice would be appreciated. How do I get my hands on this product?
Thanks again.
Leon
Hello Mister Swanepoel, please fill out our contact form on the website. The colleagues should be able to help. Here it is: https://www.uvex-safety.com/en/uvex-safety-group/how-to-contact-us/contact-form/
Thanks for sharing the post.